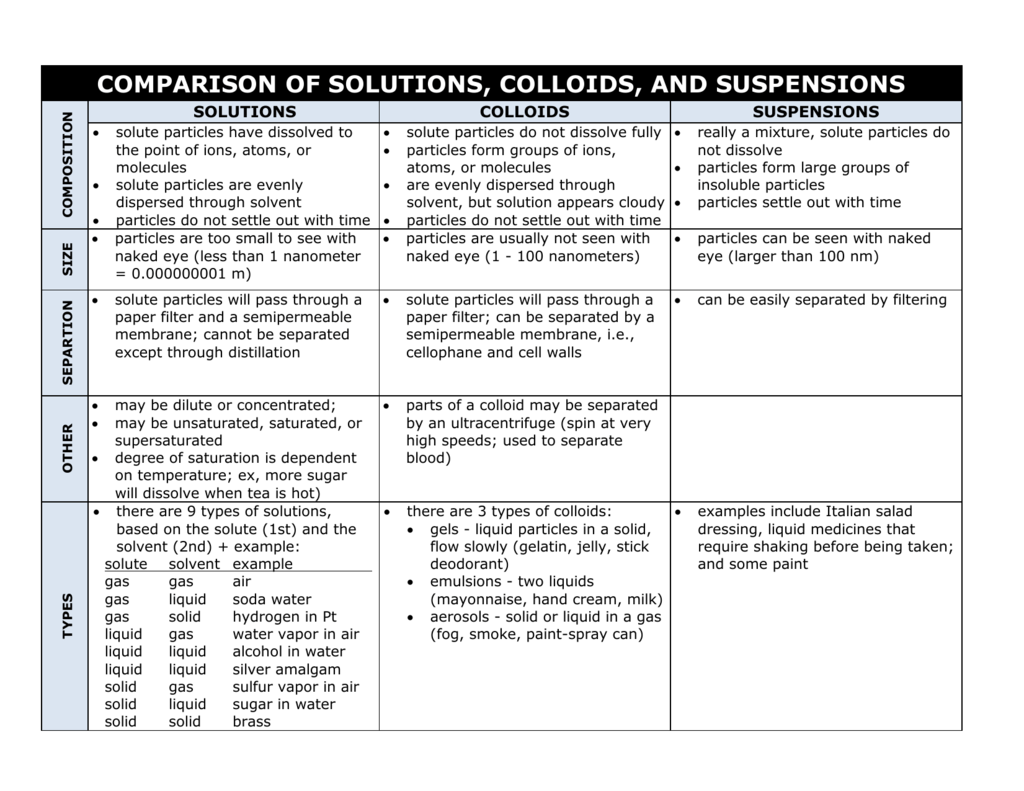
Colloid Solution Example . a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. These particles can be solid, liquid, or gas. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. examples of colloids and how to tell them from solutions and suspensions. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically.
from studylib.net
a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. These particles can be solid, liquid, or gas. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. examples of colloids and how to tell them from solutions and suspensions. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as.
Solutions, Colloids, Suspension
Colloid Solution Example a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. These particles can be solid, liquid, or gas. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. examples of colloids and how to tell them from solutions and suspensions.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Colloid Solution Example a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. in this chapter, we will consider the nature of solutions, and. Colloid Solution Example.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free Colloid Solution Example colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. These particles can be solid, liquid, or gas. a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still. Colloid Solution Example.
From chemistry-examples-00.blogspot.com
11 EXAMPLES OF COLLOIDS FOR IV FLUIDS, FOR EXAMPLES COLLOIDS OF IV Colloid Solution Example These particles can be solid, liquid, or gas. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution. Colloid Solution Example.
From sciencenotes.org
What Is a Colloid? Definition and Examples Colloid Solution Example a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers. Colloid Solution Example.
From www.youtube.com
Solution Suspension Colloid YouTube Colloid Solution Example a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. These particles can be solid,. Colloid Solution Example.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Colloid Solution Example a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. examples of colloids and how to tell them from solutions and suspensions. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloidal solution is heterogeneous solution which contains. Colloid Solution Example.
From www.brainkart.com
Classifications of Colloidal solution Surface Chemistry Colloid Solution Example a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a. Colloid Solution Example.
From www.e-streetlight.com
Solutions Colloids And Suspensions Worksheet E Street Light Colloid Solution Example These particles can be solid, liquid, or gas. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. examples of colloids and how to tell them from solutions and suspensions. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. a colloidal gold sol results. Colloid Solution Example.
From alanabbaguilar.blogspot.com
Crystalloid and Colloid Fluids AlanabbAguilar Colloid Solution Example colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. colloidal solution is heterogeneous. Colloid Solution Example.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Colloid Solution Example a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent. Colloid Solution Example.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Colloid Solution Example a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. These particles can be solid, liquid, or gas. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. a colloidal solution typically consists of particles ranging in size. Colloid Solution Example.
From pt.slideshare.net
Solutions, suspensions, and colloids Colloid Solution Example a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. These particles can be solid, liquid, or gas. colloids, also known. Colloid Solution Example.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Colloid Solution Example examples of colloids and how to tell them from solutions and suspensions. a colloidal solution typically consists of particles ranging in size from 1 nanometer to 1 micrometer. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. in this chapter, we will consider the nature of solutions, and examine factors that. Colloid Solution Example.
From www.slideserve.com
PPT Bonding PowerPoint Presentation, free download ID3050946 Colloid Solution Example colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. These particles can be solid, liquid, or gas. a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. colloids, also known as colloidal solutions or colloidal systems, are. Colloid Solution Example.
From webapi.bu.edu
Properties of colloidal solution. Colloidal System. 20221020 Colloid Solution Example examples of colloids and how to tell them from solutions and suspensions. a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and.. Colloid Solution Example.
From www.logiota.com
Preparation and Properties of Colloids Surface Chemistry Physical Colloid Solution Example a colloid is a mixture that has particles ranging between 1 and 1000 nanometers in diameter, yet are still able to remain evenly distributed. These particles can be solid, liquid, or gas. in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. colloids, also known as. Colloid Solution Example.
From ar.inspiredpencil.com
Examples Of Colloid Mixtures Colloid Solution Example in this chapter, we will consider the nature of solutions, and examine factors that determine whether a solution will form and. These particles can be solid, liquid, or gas. colloids, also known as colloidal solutions or colloidal systems, are mixtures in which microscopically. examples of colloids and how to tell them from solutions and suspensions. a. Colloid Solution Example.
From www.slideshare.net
Colloids Colloid Solution Example a colloidal gold sol results from the reduction of a very dilute solution of gold(iii) chloride by a reducing agent such as. These particles can be solid, liquid, or gas. colloidal solution is heterogeneous solution which contains particles of intermediate size between the true solution and the. colloids, also known as colloidal solutions or colloidal systems, are. Colloid Solution Example.